Disclaimer
The alfapump® system is currently not approved in Canada for commercial use. DSR® therapy is still in development and is currently not approved in the United States or Canada. Any statements regarding safety and efficacy arise from ongoing pre-clinical and clinical investigations which have yet to be completed. Sequana Medical makes no claims of safety or effectiveness of the DSR® therapy in the U.S. or Canada. There is no link between the DSR® therapy and ongoing investigations with the alfapump® system in Europe, the United States or Canada.
Ascites is the accumulation of excess fluid in the abodomen, which is a key complication of liver cirrhosis (“liver ascites”) and is also caused by certain late-stage cancers (“malignant ascites”). Ascites has a dramatic negative impact on patient quality of life including difficulties eating, moving, breathing and sleeping.
In its early stages, ascites can usually be treated with medication (diuretics) and/or a salt-restricted diet.
However, these treatments sometimes become ineffective or are no longer tolerated by the body. In such cases the ascites is described as being ‘refractory’ to medical therapy and it is removed by paracentesis or sometimes by the placement of a shunt called TIPS . If above treatments are ineffective, liver transplantation may be required.
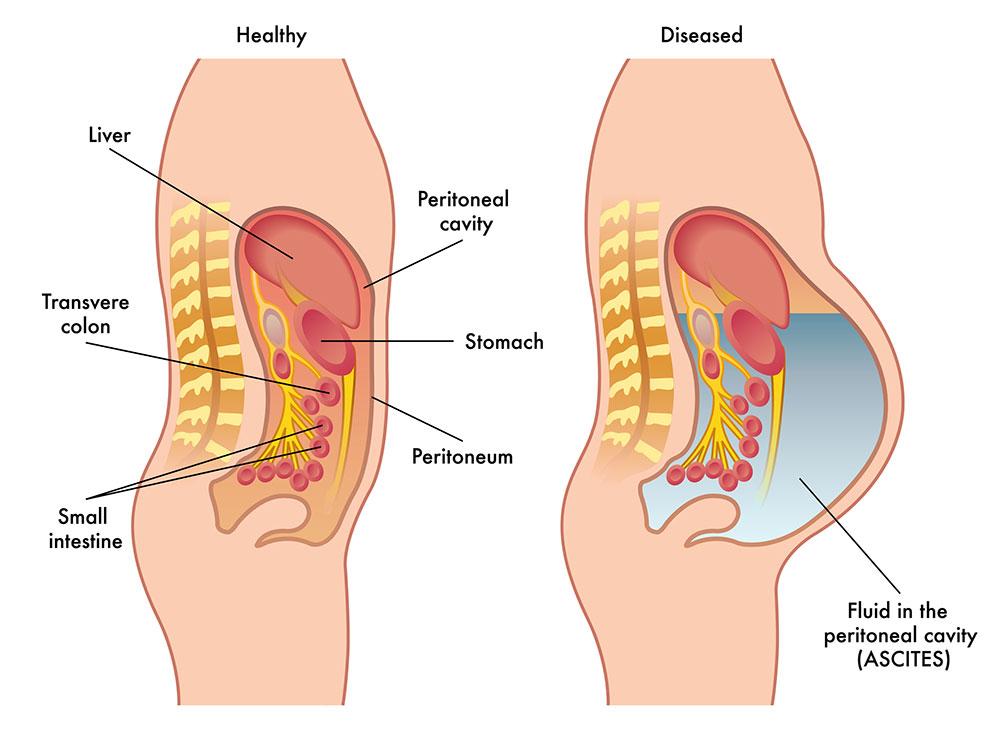
Liver ascites is the protein-containing fluid that leaks from the liver as a result of advanced cirrhosis.
Malignant ascites is ascites which is caused by certain types of advanced cancer, including ovarian, breast, pancreatic, lung, liver, colon/rectum and lymphoma cancer.
Refractory ascites is ascites that is unresponsive to a sodium-restricted diet and high-dose diuretic treatment or that recurs rapidly after medical therapy.
Management of ascites is based on a low-sodium diet and diuretic treatment. Until the alfapump®, treatment options for refractory ascites have been limited.