Disclaimer
The alfapump® system is currently not approved in Canada for commercial use. DSR® therapy is still in development and is currently not approved in the United States or Canada. Any statements regarding safety and efficacy arise from ongoing pre-clinical and clinical investigations which have yet to be completed. Sequana Medical makes no claims of safety or effectiveness of the DSR® therapy in the U.S. or Canada. There is no link between the DSR® therapy and ongoing investigations with the alfapump® system in Europe, the United States or Canada.
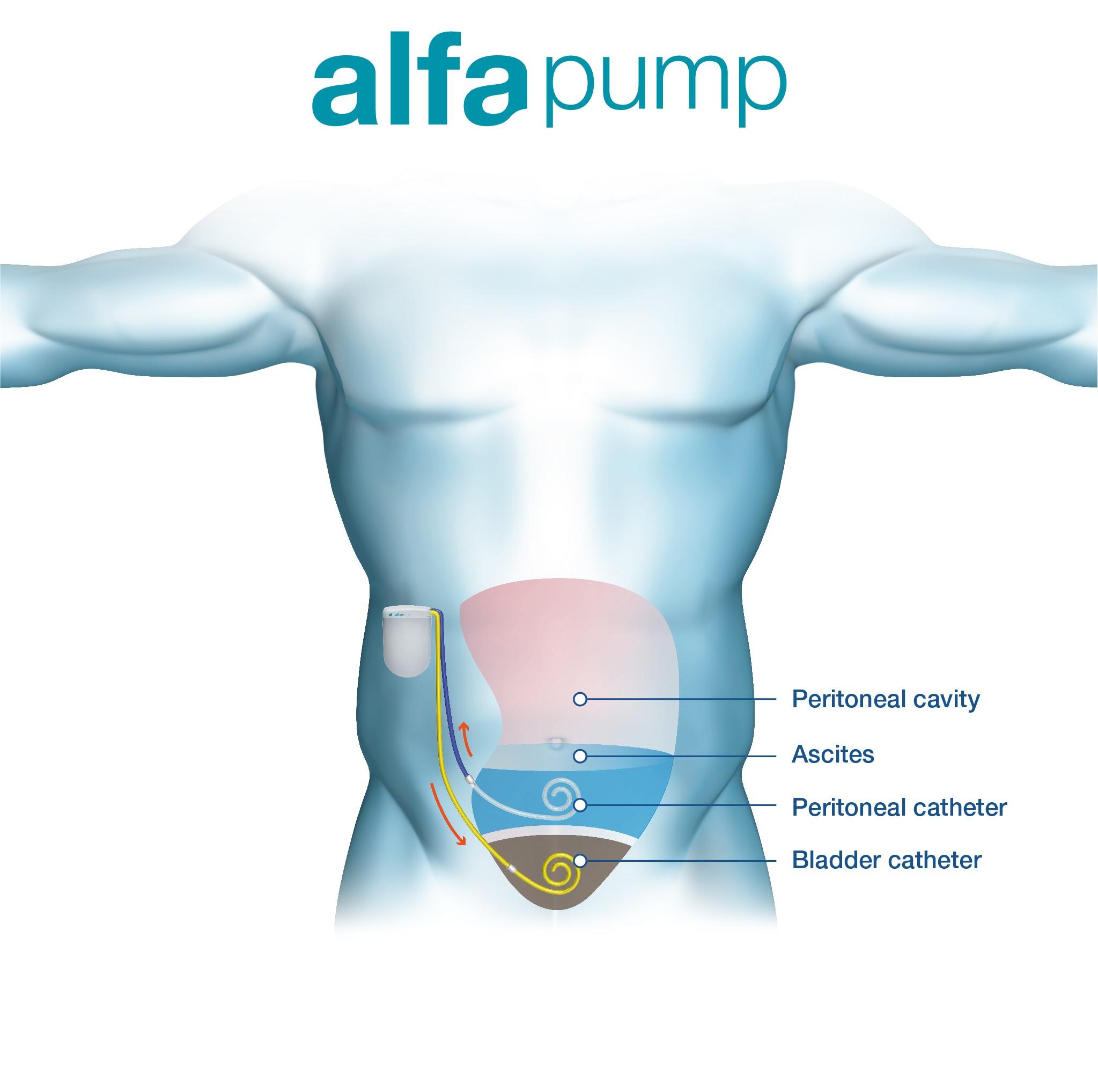
The alfapump® is a fully-implantable, wirelessly-charged, CE-marked system that automatically and continuously pumps ascites from the abdominal cavity into the bladder, where the body eliminates the ascites naturally.
The alfapump® system’s DirectLink Technology allows physicians to monitor pump performance and more effectively manage their patients.
Since 2018, the alfapump® has been included in the EASL (European Association for the Study of the Liver) clinical practice guidelines.
The ascites is removed from the body on a continual basis, thereby preventing fluid build-up in the abdomen.
The alfapump® is programmed by the patient’s doctor allow an optimal removal of ascites on a daily basis, customised to the patient’s needs.
The alfapump® has a special mechanism which ensures ascites is only moved into the bladder if there is space and it will turn itself off if there is no ascites in the abdominal cavity, ensuring optimal fluid management with no inconvenience for the patient.